Thermodynamic Database
Components (21)
Total of 21 components are included in the database as listed here:
Major alloying elements: Ag, Al, Co, Cr, Cu, Fe, Hf, Li, Mg, Mn, Mo, Ni, O, Si, Sn, Ti, V, Zn and Zr
Minor alloying element: B, C
Suggested Composition Range
The suggested composition range for each element is listed in Table 1.
| Elements | Composition Range (wt.%) |
|---|---|
|
Ag, Al, Co, Cr, Cu, Fe, HF, Li, Mg, Mn, Mo, Ni, Si, Sn, Ti, V, Zn, Zr |
0 ~ 100 |
|
B, C, O |
0 ~ 10 |
What is new in PanHEA2024
-
Addition of the component O
-
Improvement of the Al-Ni and Mn-Ti binary descriptions
Phases
Total of 687 phases are included in the current database. The names and thermodynamic models of some phases are given in Table 2. Information on all the other phases is listed in PanAl2024: List of Phases. Users can also view it through TDB viewer of Pandat™ .
Assessed Subsystems
A total of 478 systems, including 203 binary and 275 ternary subsystems have been assessed. The modeling status is indicated by numbers. The systems with number 10 are fully assessed in the whole composition range. The higher value shows higher reliability of the system.
Binary Systems (203)
| Ag-Al(10) | Ag-B(10) | Ag-C(10) | Ag-Co(10) | Ag-Cr(10) | Ag-Cu(10) | Ag-Fe(10) |
| Ag-Mg(10) | Ag-Mn(10) | Ag-Mo(10) | Ag-Ni(10) | Ag-O(5) | Ag-Si(10) | Ag-Sn(10) |
| Ag-Ti(10) | Ag-V(10) | Ag-Zn(10) | Ag-Zr(10) | Al-B(10) | Al-C(10) | Al-Co(10) |
| Al-Cr(10) | Al-Cu(10) | Al-Fe(10) | Al-Hf(10) | Al-Li(10) | Al-Mg(10) | Al-Mn(10) |
| Al-Mo(10) | Al-Ni(10) | Al-O(10) | Al-Si(10) | Al-Sn(10) | Al-Ti(10) | Al-V(10) |
| Al-Zn(10) | Al-Zr(10) | B-C(10) | B-Co(10) | B-Cr(10) | B-Cu(10) | B-Fe(10) |
| B-Hf(10) | B-Mg(10) | B-Mn(10) | B-Mo(10) | B-Ni(10) | B-O(10) | B-Si(10) |
| B-Sn(10) | B-Ti(10) | B-V(10) | B-Zn(10) | B-Zr(10) | C-Co(10) | C-Cr(10) |
| C-Fe(10) | C-Hf(10) | C-Li(10) | C-Mg(10) | C-Mn(10) | C-Mo(10) | C-Ni(10) |
| C-O(10) | C-Si(10) | C-Sn(10) | C-Ti(10) | C-V(10) | C-Zn(10) | C-Zr(10) |
| Co-Cr(10) | Co-Cu(10) | Co-Fe(10) | Co-Hf(10) | Co-Li(10) | Co-Mg(10) | Co-Mn(10) |
| Co-Mo(10) | Co-Ni(10) | Co-O(10) | Co-Si(10) | Co-Sn(10) | Co-Ti(10) | Co-V(10) |
| Co-Zn(10) | Co-Zr(10) | Cr-Cu(10) | Cr-Fe(10) | Cr-Hf(10) | Cr-Li(10) | Cr-Mg(10) |
| Cr-Mn(10) | Cr-Mo(10) | Cr-Ni(10) | Cr-O(10) | Cr-Si(10) | Cr-Sn(10) | Cr-Ti(10) |
| Cr-V(10) | Cr-Zn(10) | Cr-Zr(10) | Cu-Fe(10) | Cu-Hf(10) | Cu-Li(10) | Cu-Mg(10) |
| Cu-Mn(10) | Cu-Mo(10) | Cu-Ni(10) | Cu-O(10) | Cu-Si(10) | Cu-Sn(10) | Cu-Ti(10) |
| Cu-V(10) | Cu-Zn(10) | Cu-Zr(10) | Fe-Hf(10) | Fe-Li(10) | Fe-Mg(10) | Fe-Mn(10) |
| Fe-Mo(10) | Fe-Ni(10) | Fe-O(10) | Fe-Si(10) | Fe-Sn(10) | Fe-Ti(10) | Fe-V(10) |
| Fe-Zn(10) | Fe-Zr(10) | Hf-Li(10) | Hf-Mg(10) | Hf-Mn(10) | Hf-Mo(10) | Hf-Ni(10) |
| Hf-O(10) | Hf-Si(10) | Hf-Sn(10) | Hf-Ti(10) | Hf-V(10) | Hf-Zr(10) | Li-Mg(10) |
| Li-Mn(10) | Li-Ni(10) | Li-O(10) | Li-Si(10) | Li-Sn(10) | Li-Ti(10) | Li-V(10) |
| Li-Zn(10) | Li-Zr(10) | Mg-Mn(10) | Mg-Mo(10) | Mg-Ni(10) | Mg-O(10) | Mg-Si(10) |
| Mg-Sn(10) | Mg-Ti(10) | Mg-V(10) | Mg-Zn(10) | Mg-Zr(10) | Mn-Mo(10) | Mn-Ni(10) |
| Mn-O(10) | Mn-Si(10) | Mn-Sn(10) | Mn-Ti(10) | Mn-V(10) | Mn-Zn(10) | Mn-Zr(10) |
| Mo-Ni(10) | Mo-O(10) | Mo-Si(10) | Mo-Sn(10) | Mo-Ti(10) | Mo-V(10) | Mo-Zr(10) |
| Ni-O(10) | Ni-Si(10) | Ni-Sn(10) | Ni-Ti(10) | Ni-V(10) | Ni-Zn(10) | Ni-Zr(10) |
| O-Si(10) | O-Sn(10) | O-Ti(10) | O-V(10) | O-Zn(5) | O-Zr(10) | Si-Sn(10) |
| Si-Ti(10) | Si-V(10) | Si-Zn(10) | Si-Zr(10) | Sn-Ti(10) | Sn-V(10) | Sn-Zn(10) |
| Sn-Zr(10) | Ti-V(10) | Ti-Zn(10) | Ti-Zr(10) | V-Zn(10) | V-Zr(10) | Zn-Zr(10) |
Ternary Systems (275)
| Ag-Al-Co(5) | Ag-Al-Cu(10) | Ag-Al-Fe(10) | Ag-Al-Mg(10) | Ag-Al-Si(10) | Ag-Al-Sn(10) |
| Ag-Al-Zn(10) | Ag-Co-Sn(5) | Ag-Cr-Cu(10) | Ag-Cu-Mg(10) | Ag-Cu-Ni(10) | Ag-Cu-Si(10) |
| Ag-Cu-Sn(10) | Ag-Cu-Ti(10) | Ag-Cu-Zr(10) | Ag-Ni-Sn(10) | Ag-Sn-Ti(5) | Al-Co-Cr(10) |
| Al-Co-Cu(10) | Al-Co-Fe(10) | Al-Co-Mg(10) | Al-Co-Mn(10) | Al-Co-Mo(10) | Al-Co-Ni(10) |
| Al-Co-Si(10) | Al-Co-Ti(10) | Al-Co-Zr(10) | Al-Cr-Cu(10) | Al-Cr-Fe(10) | Al-Cr-Mg(10) |
| Al-Cr-Mn(10) | Al-Cr-Mo(10) | Al-Cr-Ni(10) | Al-Cr-Si(10) | Al-Cr-Ti(10) | Al-Cr-Zr(10) |
| Al-Cu-Fe(10) | Al-Cu-Li(10) | Al-Cu-Mg(10) | Al-Cu-Mn(10) | Al-Cu-Mo(10) | Al-Cu-Ni(10) |
| Al-Cu-Si(10) | Al-Cu-Sn(10) | Al-Cu-Ti(10) | Al-Cu-V(10) | Al-Cu-Zn(10) | Al-Cu-Zr(10) |
| Al-Fe-Mg(8) | Al-Fe-Mn(10) | Al-Fe-Mo(8) | Al-Fe-Ni(10) | Al-Fe-Si(10) | Al-Fe-Ti(10) |
| Al-Fe-V(10) | Al-Fe-Zn(10) | Al-Fe-Zr(10) | Al-Li-Mg(10) | Al-Li-Mn(10) | Al-Li-Si(10) |
| Al-Li-Zr(10) | Al-Mg-Mn(10) | Al-Mg-Mo(8) | Al-Mg-Si(10) | Al-Mg-Sn(10) | Al-Mg-Ti(10) |
| Al-Mg-Zn(10) | Al-Mn-Ni(5) | Al-Mn-Si(10) | Al-Mn-Ti(10) | Al-Mn-Zn(10) | Al-Mo-Ni(10) |
| Al-Mo-Si(10) | Al-Mo-Ti(10) | Al-Mo-V(10) | Al-Mo-Zr(10) | Al-Ni-Si(10) | Al-Ni-Ti(10) |
| Al-Ni-V(10) | Al-Ni-Zn(10) | Al-Ni-Zr(10) | Al-Si-Sn(10) | Al-Si-Ti(10) | Al-Si-V(10) |
| Al-Si-Zn(10) | Al-Si-Zr(10) | Al-Sn-Ti(10) | Al-Ti-V(10) | Al-Ti-Zn(10) | Al-Ti-Zr(10) |
| Al-V-Zr(10) | C-Mo-Zr(10) | Co-Cr-Cu(10) | Co-Cr-Fe(10) | Co-Cr-Mn(10) | Co-Cr-Mo(10) |
| Co-Cr-Ni(10) | Co-Cr-Si(10) | Co-Cr-Ti(10) | Co-Cr-V(10) | Co-Cr-Zr(10) | Co-Cu-Fe(10) |
| Co-Cu-Hf(10) | Co-Cu-Mn(10) | Co-Cu-Mo(10) | Co-Cu-Ni(10) | Co-Cu-Ti(10) | Co-Fe-Mn(10) |
| Co-Fe-Mo(10) | Co-Fe-Ni(10) | Co-Fe-Si(10) | Co-Fe-Ti(10) | Co-Fe-V(10) | Co-Fe-Zr(10) |
| Co-Hf-Zr(10) | Co-Mn-Mo(10) | Co-Mn-Ni(10) | Co-Mn-Si(10) | Co-Mn-Ti(10) | Co-Mn-Zr(10) |
| Co-Mo-Ni(10) | Co-Mo-Ti(10) | Co-Mo-Zr(10) | Co-Ni-Si(10) | Co-Ni-Ti(10) | Co-Ni-V(10) |
| Co-Si-Ti(10) | Co-Si-V(10) | Co-Si-Zr(10) | Co-Sn-Ti(10) | Co-V-Zr(10) | Cr-Cu-Fe(10) |
| Cr-Cu-Mn(10) | Cr-Cu-Mo(5) | Cr-Cu-Ni(10) | Cr-Cu-Sn(10) | Cr-Cu-Ti(10) | Cr-Cu-Zr(10) |
| Cr-Fe-Mg(10) | Cr-Fe-Mn(10) | Cr-Fe-Mo(10) | Cr-Fe-Ni(10) | Cr-Fe-Si(10) | Cr-Fe-Sn(10) |
| Cr-Fe-Ti(10) | Cr-Fe-V(10) | Cr-Fe-Zr(10) | Cr-Mg-Ni(10) | Cr-Mn-Ni(10) | Cr-Mn-Si(10) |
| Cr-Mn-Ti(10) | Cr-Mn-V(10) | Cr-Mn-Zr(10) | Cr-Mo-Ni(10) | Cr-Mo-Si(10) | Cr-Mo-Ti(10) |
| Cr-Mo-V(10) | Cr-Mo-Zr(10) | Cr-Ni-Si(10) | Cr-Ni-Ti(10) | Cr-Ni-V(10) | Cr-Ni-Zr(10) |
| Cr-Si-Ti(10) | Cr-Sn-Ti(10) | Cr-Sn-Zr(5) | Cr-Ti-V(10) | Cr-Ti-Zr(10) | Cr-V-Zr(10) |
| Cu-Fe-Mg(8) | Cu-Fe-Mn(10) | Cu-Fe-Mo(10) | Cu-Fe-Ni(10) | Cu-Fe-Si(10) | Cu-Fe-Sn(10) |
| Cu-Fe-Ti(10) | Cu-Fe-V(10) | Cu-Fe-Zn(8) | Cu-Fe-Zr(10) | Cu-Hf-Ni(10) | Cu-Mg-Mn(8) |
| Cu-Mg-Ni(10) | Cu-Mg-Si(10) | Cu-Mg-Sn(10) | Cu-Mg-Ti(10) | Cu-Mg-Zn(10) | Cu-Mg-Zr(10) |
| Cu-Mn-Ni(10) | Cu-Mn-Si(10) | Cu-Mn-Sn(10) | Cu-Mn-Ti(10) | Cu-Mn-Zn(10) | Cu-Ni-Si(10) |
| Cu-Ni-Sn(10) | Cu-Ni-Ti(10) | Cu-Ni-V(10) | Cu-Ni-Zr(10) | Cu-Si-Sn(10) | Cu-Si-Ti(10) |
| Cu-Si-Zn(10) | Cu-Sn-Ti(10) | Cu-Ti-Zr(10) | Fe-Mg-Mn(5) | Fe-Mg-Si(10) | Fe-Mg-Zn(5) |
| Fe-Mg-Zr(10) | Fe-Mn-Mo(10) | Fe-Mn-Ni(10) | Fe-Mn-Si(10) | Fe-Mn-Ti(10) | Fe-Mn-V(10) |
| Fe-Mn-Zn(5) | Fe-Mn-Zr(10) | Fe-Mo-Ni(10) | Fe-Mo-Si(5) | Fe-Mo-Ti(10) | Fe-Mo-V(10) |
| Fe-Mo-Zr(10) | Fe-Ni-Si(10) | Fe-Ni-Ti(10) | Fe-Ni-V(10) | Fe-Ni-Zn(10) | Fe-Ni-Zr(10) |
| Fe-Si-Sn(10) | Fe-Si-Ti(10) | Fe-Si-V(10) | Fe-Si-Zn(10) | Fe-Si-Zr(10) | Fe-Sn-Zr(10) |
| Fe-Ti-V(10) | Fe-Ti-Zn(10) | Fe-Ti-Zr(10) | Hf-Ni-Ti(10) | Hf-Ni-Zr(5) | Hf-Ti-Zr(10) |
| Li-Mg-Si(10) | Li-Mg-Zr(10) | Mg-Mn-Ni(10) | Mg-Mn-Si(10) | Mg-Mn-Zn(8) | Mg-Ni-Si(10) |
| Mg-Ni-Zn(10) | Mg-Si-Sn(10) | Mg-Si-Zn(10) | Mg-Si-Zr(10) | Mn-Mo-Ni(10) | Mn-Mo-Ti(10) |
| Mn-Ni-Si(10) | Mn-Ni-Ti(10) | Mn-Si-Sn(10) | Mn-Si-Ti(10) | Mn-Si-Zn(5) | Mn-Ti-V(10) |
| Mn-Ti-Zr(10) | Mo-Ni-Si(10) | Mo-Ni-Ti(10) | Mo-Ni-V(10) | Mo-Ni-Zr(10) | Mo-Si-Ti(10) |
| Mo-Si-V(10) | Mo-Si-Zr(10) | Mo-Ti-V(10) | Mo-Ti-Zr(10) | Mo-V-Zr(10) | Ni-Si-Ti(10) |
| Ni-Si-V(10) | Ni-Si-Zn(10) | Ni-Si-Zr(10) | Ni-Sn-Ti(10) | Ni-Sn-Zr(10) | Ni-Ti-V(10) |
| Ni-Ti-Zr(10) | Ni-V-Zr(10) | Si-Ti-V(10) | Sn-Ti-Zr(10) | Ti-V-Zr(10) |
Database Applications
The PanHEA database is validated by a large amount of experimental data. It is by far the most comprehensive database for alloy design and processing optimization of high entropy alloys for 3rd-transition metals. A few example calculations using the current PanHEA database are given below:
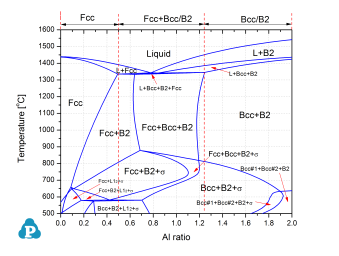
Figure 1 shows the calculated isopleth of the AlxCoCrFeNi system with x varies between 0~2. x is the Al ratio and other elements are in the equal atomic percent. It indicates that Al is a strong stabilizer for both the disorder Bcc and ordered B2 structure, and the later is favored. In addition to the phase equilibrium information with the changing of Al ratio at different temperatures, one can also predict the as-cast microstructures of the HEAs based on the calculated mushy-zone (Liquid + Solid) phase regions (as indicated on top of this figure). It shows that pure Fcc structure will be developed with lower Al ratio, while Bcc structure (Bcc and/or B2) will be developed with higher Al ratio, and a mixture of Fcc+Bcc should be seen in between. Our predictions are exactly what were observed by many researchers who have carried our experimental studies of AlxCoCrFeNi alloys [2006Ke, 2008Wan, 2011Kao].
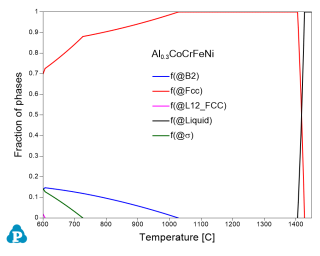
For quantitative analysis on the phase transformations of an alloy at the given composition, the equilibrium calculation is needed. Figure 2 shows equilibrium calculation of the Al0.3CoCrFeNi alloy at the temperature range from 600 to 1450 °C. It indicates that this alloy contains single Fcc phase at higher temperatures (>1000 °C). The B2 phase precipitates out at around 1000 °C. At lower temperatures (<700 °C), other phases will form as well, such as σ and L12_Fcc. The alloy with Fcc structure shows good ductility but low strength, while the B2-type intermetallic compounds generally show excellent strength but low ductility. Usually, an HEA with dispersed B2 precipitates within the Fcc matrix has balanced properties and good performance. On the basis of thermodynamic calculations (as shown in Figure 1.2), the Al0.3CoCrFeNi HEA may meet the requirements. As shown in Figure 2, the solvus temperature of B2 is about 1060 °C. When annealing below this temperature, the B2 precipitates will form. Shu et al. [2009Shu] investigated the microstructure and tensile behaviors of the Al0.3CoCrFeNi HEA, and they found that the as-cast structure of this alloy is Fcc and the B2 phase formed when aged at 900 °C for 72h. The tensile strength of this alloy after heat treatment was significantly improved. Prediction from our database agrees with their experimental observation very well.
[2006Ke] G.Y. Ke, S.K. Chen, T. Hsu, J.W. Yeh, Annales de Chimie-Science Des Matériaux, 31(6) (2006): 669-684.
[2008Wan] Y.P. Wang, B.S. Li, M.X. Ren, C. Yang, H.Z. Fu, Mat. Sci. Eng. A, 491(1-2) (2008): 154-158.
[2009Shu] T.T. Shun, Y.C. Du, J Alloys Compd., 479 (2009): 157-160.
[2011Kao] Y.F. Kao, S.K. Chen, T.J. Chen, P.C. Chu, J.W. Yeh,, S.J. Lin, J Alloys Compd., 509 (2011): 1607-1614.
[2018Lap] G. Laplanche, et al., Elastic moduli and thermal expansion coefficients of medium-entropy subsystems of the CrMnFeCoNi high-entropy alloy. Journal of Alloys and Compounds, 746 (2018): 244-255.